Sectieoverzicht
-
-
Cohesion is the attractive force acting between two portions of a substance which are next to each other. This is usually found in a solid or liquid. It is this force that holds a piece of matter together.
Forces act also between two unlike substances in contact, which is called adhesion. These forces come from electrical forces. The forces of cohesion and adhesion act over a short range and vary in strength, depending on the substances concerned.
Cohesive Forces are attractive forces between molecules of the same type.
Adhesive Forces are attractive forces between different types of molecules.

Figure 1: Cohesion is a force which acts between molecules and adhesion is a force which acts between two different substances.
Here are examples of adhesion:
Honey sticking to your fingers
Water drops sticking to a window without falling
Water wetting a plastic cutting board

Figure 2: Mercury wetting a surface

Figure 3: Dewdrops clinging to a leaf tip

Figure 4: Tape sticking to paper

Figure 5: Waxing a car. Droplets of water on a freshly waxed car do not wet the car well because of low adhesion between water and the waxed surface. This helps protect the car from rust.
Cohesion occurs when atoms or molecules experience attraction to themselves. This occurs within some compounds and in metals.
Here are examples of cohesion:
Water forming beads on a window

Figure 6: Water striders floating on water
Part of the reason people have trouble keeping adhesion and cohesion straight is because both processes occur at once. For example, a water drop sticking to a window shows both adhesion and cohesion.
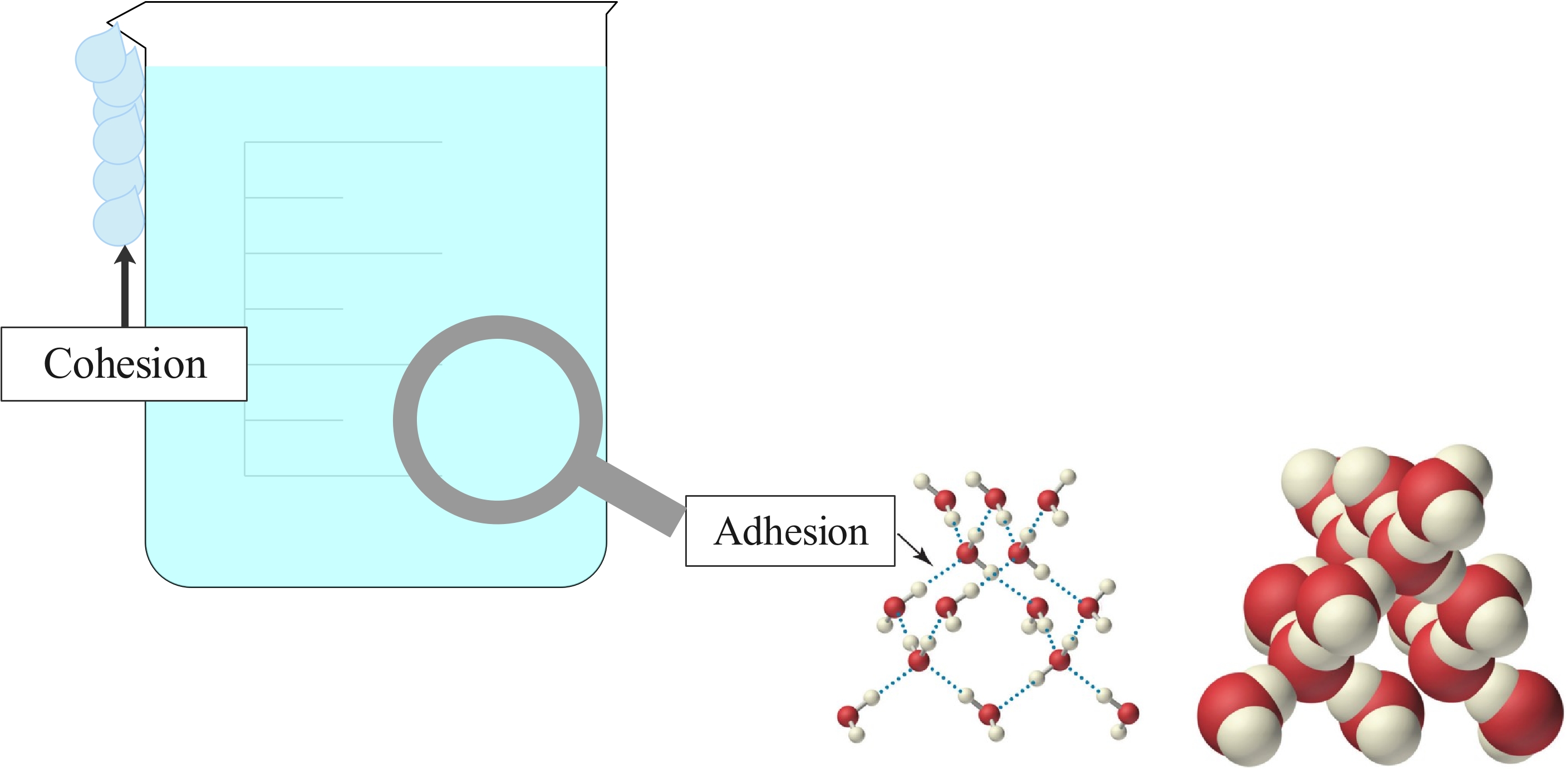
Figure 7: Water's ability to form a rounded shape is an example of cohesion. Water droplets sticking to the glass instead of falling right away demonstrates adhesion.
A meniscus is the curved line that forms at the upper surface of a liquid in a container. The shape of the meniscus depends on the difference between the adhesion between the liquid and the container surface and the cohesion between the liquid molecules.
A concave meniscus forms when the liquid is more attracted to its container than it is to itself. In other words, adhesion is greater than cohesion. Water in a glass tube has a concave meniscus, even though it has high cohesion.
A convex meniscus forms when the liquid is more attracted to itself than to its container. Cohesion is greater than adhesion. Mercury in a glass container has a convex meniscus.

Figure 8: the concave meniscus of water and the convex meniscus of mercury
A flat meniscus occurs when adhesion and cohesion are in balance. Water in a plastic container has a nearly flat meniscus.
-
