Sectieoverzicht
-
-
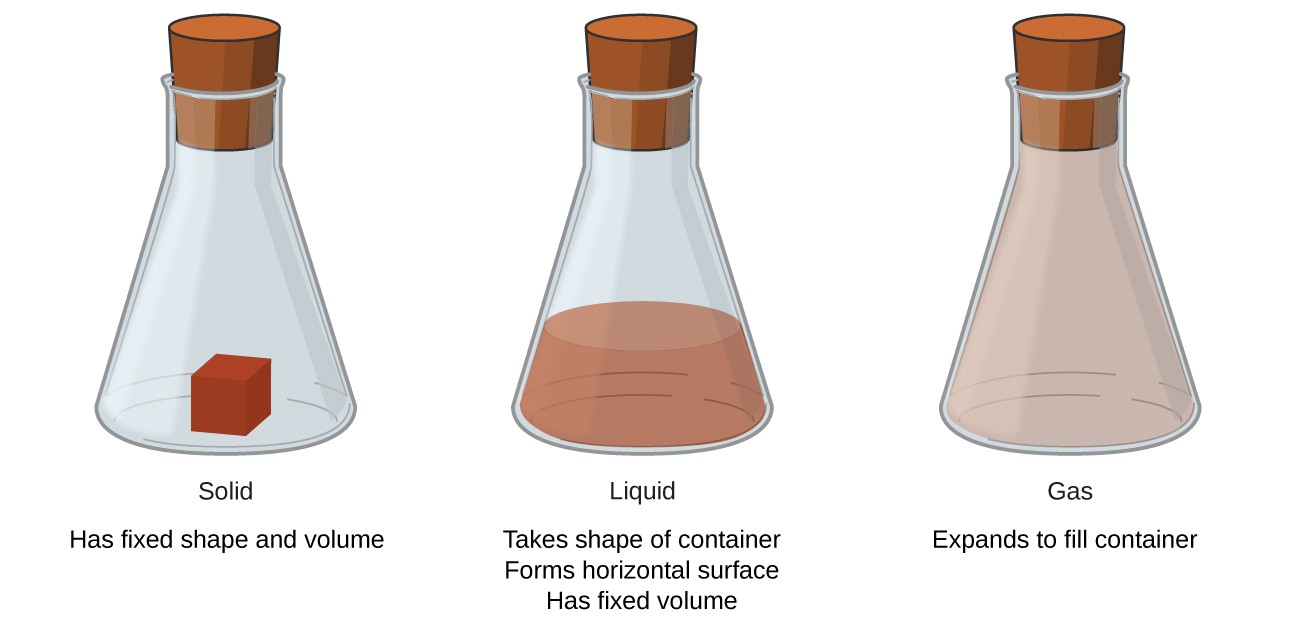
The kinetic particle theory explains the properties of solids, liquids and gases. The work kinetic means movement.
All matter consists of particles including atoms and molecules. In everyday life, there are three states of matter - solids, liquids, and gases. The differences between the three states are due to the arrangement and spacing of the particles and their motion.
Kinetic theory of matter states that “Matter is made up of those substances or particles which are constantly moving.” The energy level of the particles depends upon the temperature possessed by the matter. This helps us to determine whether that matter is in a solid, liquid, or gas state.
Activity 1: Make a model to see how particles behave
What you will need:
· a rectangular box (as big as a shoebox lid,) large enough to allow free movement for five marbles
· 5 marbles (or small hard balls of the same size) – one should be different in colour from the others
· a broad flat board that can fit into the box and can close off the end of the box (see diagram in Part A), like a small ruler
· a watch or timer, for example on a cellphone
Task 1
Place the marbles in the box on a flat surface such as a table. (Remember that your box should be large enough to allow free movement of the marbles.) Shake the box in all directions for 30 seconds, but without lifting it off the table. Then answer the questions below:

1. Describe the movement of the marbles.
2. Can you predict where a marble will move? Explain your answer.
3. Repeat this task and watch one of the marbles. List all the things it bumps against in the 30 seconds.
We call this disorganised movement shown by the marbles random motion.
In task 2, we will reduce the space in the box to find out how the marbles behave in a smaller space.
Task 2
Use the broad flat board (or a ruler) to make the space inside the box half as big, as shown here. Now shake the box as before. Answer the following questions:
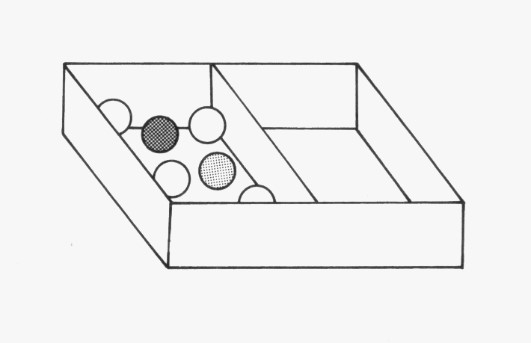
1. Compare the movement of the marbles with your observations in task 1.
2. Count the number of collisions the marble makes with its neighbouring marbles and with the sides of the box.
In task 3 we will decrease the space even further.
Task 3
Place your board such that the marbles are as tightly packed as possible, but do not squash them. Shake the box as before, and then answer the questions below:
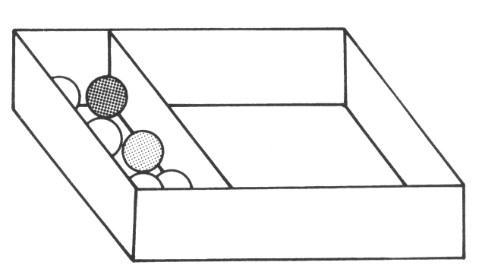
1. How do the marbles move now?
2. Shake the box faster.
3. Can you see any difference in the motion of the marbles?
4. Count the collisions of the marbles and compare the movement of the marbles now to how they moved in tasks 1 and 2 of this activity.
What did you find?
In task 1, the marbles were far apart and moved freely in a large space. This is also true of the particles in a gas. They have large spaces between them and move about freely. You would have observed, during the activity, that the particles collide with one other and with the sides of the container. There are also large spaces between the particles, on average. By 'on average' we mean there are large spaces most of the time except when they are colliding. When they are colliding, they are close together. But they have great freedom of movement.
In task 2, the particles were closer together but very loosely packed and there was some room for the marbles to move. This is like the arrangement of particles in a liquid, where the particles are close together, but they can still slide over one another.
In task 3, the marbles were fixed in position and could not move about. This arrangement is like the arrangement of particles in solids.
-
