Sectieoverzicht
-
-
The Concept of Matter
Matter is the name given to all the things around you. Matter is any substance which has mass and occupies space. All physical objects are composed of matter, in the form of atoms and molecules, which are in turn composed of protons, neutrons, and electrons.
All matter can be categorised as a solid, liquid, gas, or a plasma. This is called the states of matter.
Classification of Matter
1: Pure Substance: Matter with a consistent arrangement.
Element: comprised of just one kind of atom. Example - gold, silver, carbon, oxygen, and hydrogen
Compound: at least two different elements which are chemically joined together. For example - water, carbon dioxide, sodium bicarbonate, carbon monoxide
2: Mixed Substance: matter with a variable creation.
Heterogenous mixtures: A heterogeneous mixture is one that contains two or more substances in a different state. The substances in the mixture are not evenly distributed throughout the mixture. For example, sand and soil.
Homogeneous Mixtures: A homogeneous mixture can be a gaseous, liquid, or solid mixture that has the same proportions of its components throughout a given sample. There is only one phase of matter observed in a homogeneous mixture. For example - saltwater.
Homogenous mixtures can be further classified as:
Colloids: Colloids are mixtures in which microscopically dispersed insoluble particles of one substance are suspended in another substance. For example, flour and water.
Solutions: In a solution, all the components appear as a single phase and the particles are evenly distributed. This is why a whole bottle of soft drink has the same taste throughout.
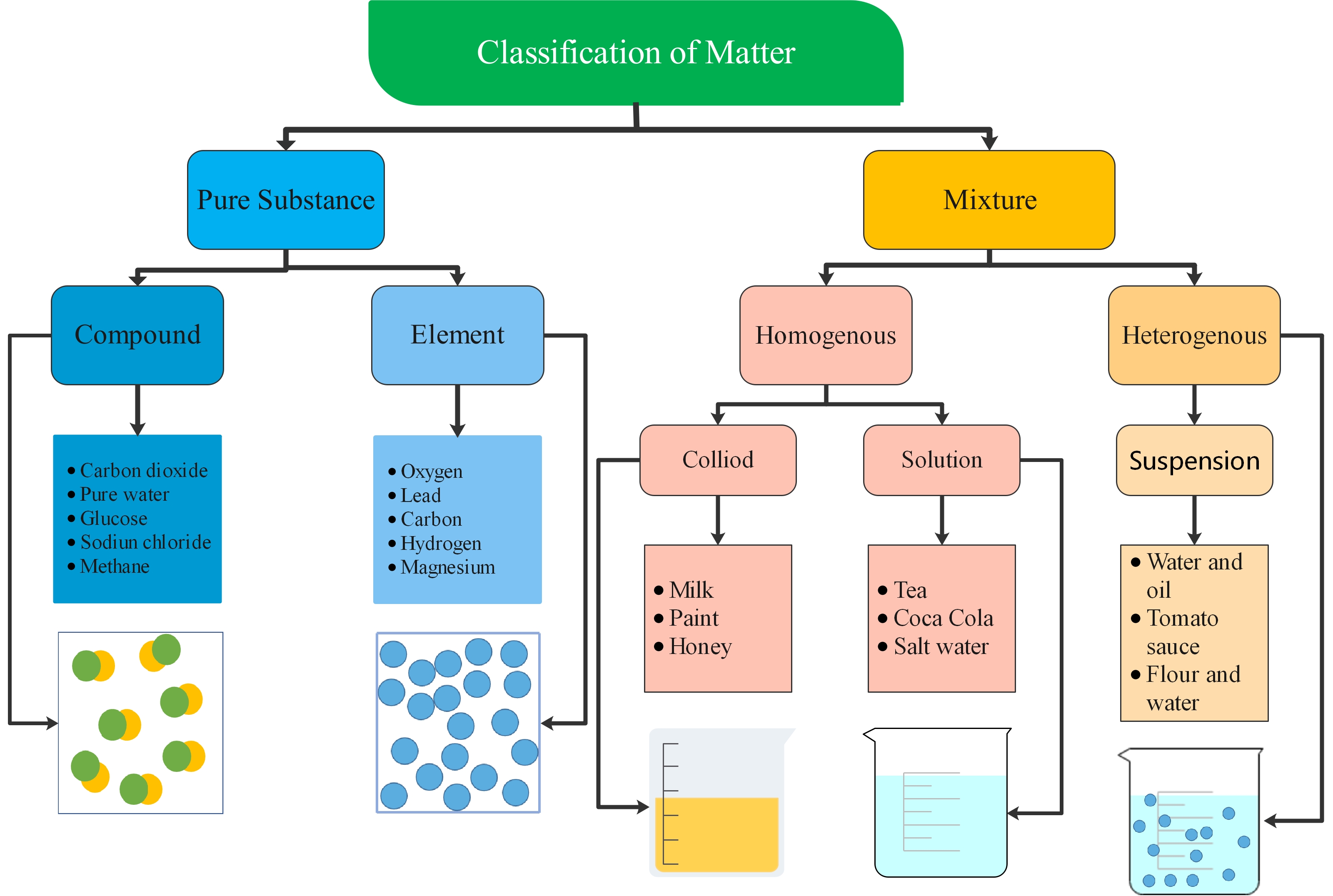
Figure 1: A concept map of the classification of matter.Characteristics of Particle Nature of Matter
The following are the characteristics of the particle nature of matter:
- Atoms and molecules are tiny particles. They are not visible to the naked eye.
- Particles always attract each other: The molecules in solids have no space as they are closely bonded by a strong force of attraction; this force is weaker in liquid and gas.
- All particles are continuously in motion. When the particles are heated, their speed changes and they begin to move quickly. The opposite is true when particles cool.
- There is space between the particles of matter. When sugar is dissolved into water, the sugar crystals separate into very fine particles. These particles of sugar occupy the spaces between the various particles of water. Hence, there is no change in the volume of water.
Brownian Motion
Activity 1
1. Place a few crystals of potassium permanganate in a glass of water. If potassium permanganate is not available, use a couple of drops of food colouring.
2. Observe.
3. Heat some water and repeat step 1. What do you notice about speed at which the potassium permanganate moves through the water?

Figure 2: The 'spreading out' of potassium permanganate in a beaker of water.
Conclusion
When a potassium permanganate crystal is placed in a water beaker, the water slowly turns purple on its own, without stirring. The particles separate each other and spread throughout the water turning the water completely purple. When the water temperature is increased, the particles spread out through the water more quickly.
What is actually happening is on dissolving, the particles of potassium permanganate get into the spaces between the particles of water.
So, it can be concluded that the particles are moving, or they are in motion.
Brownian motion refers to the random movement displayed by small particles that are suspended in fluids. This motion is a result of the collisions of the particles with other fast-moving particles in the fluid.
Brownian motion is named after the Scottish Botanist Robert Brown, who first observed that pollen grains move in random directions when placed in water.
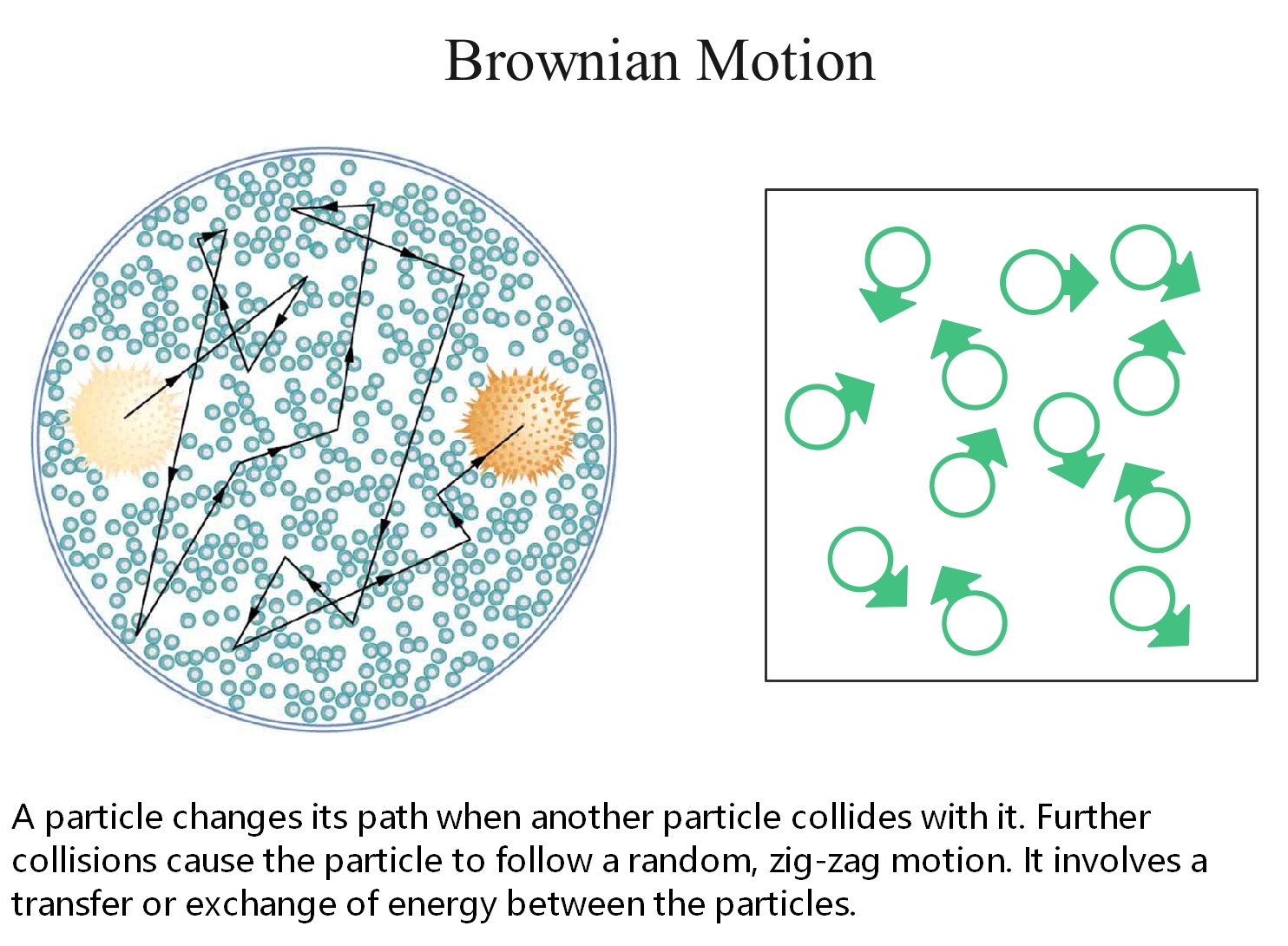
Figure 3: An illustration describing the random movement of fluid particles caused by the collisions between these particles.
-
