Sectieoverzicht
-
-
Measuring the density of different objects
There are different ways to investigate density. In this required practical activity, it is important to:
- record the mass accurately
- measure and observe the mass and the volume of the different objects
- use appropriate apparatus and methods to measure volume and mass and use that to investigate density
Aim of the experiment
To measure the density of various materials.
Method
Method 1: Stone or other irregular shaped object
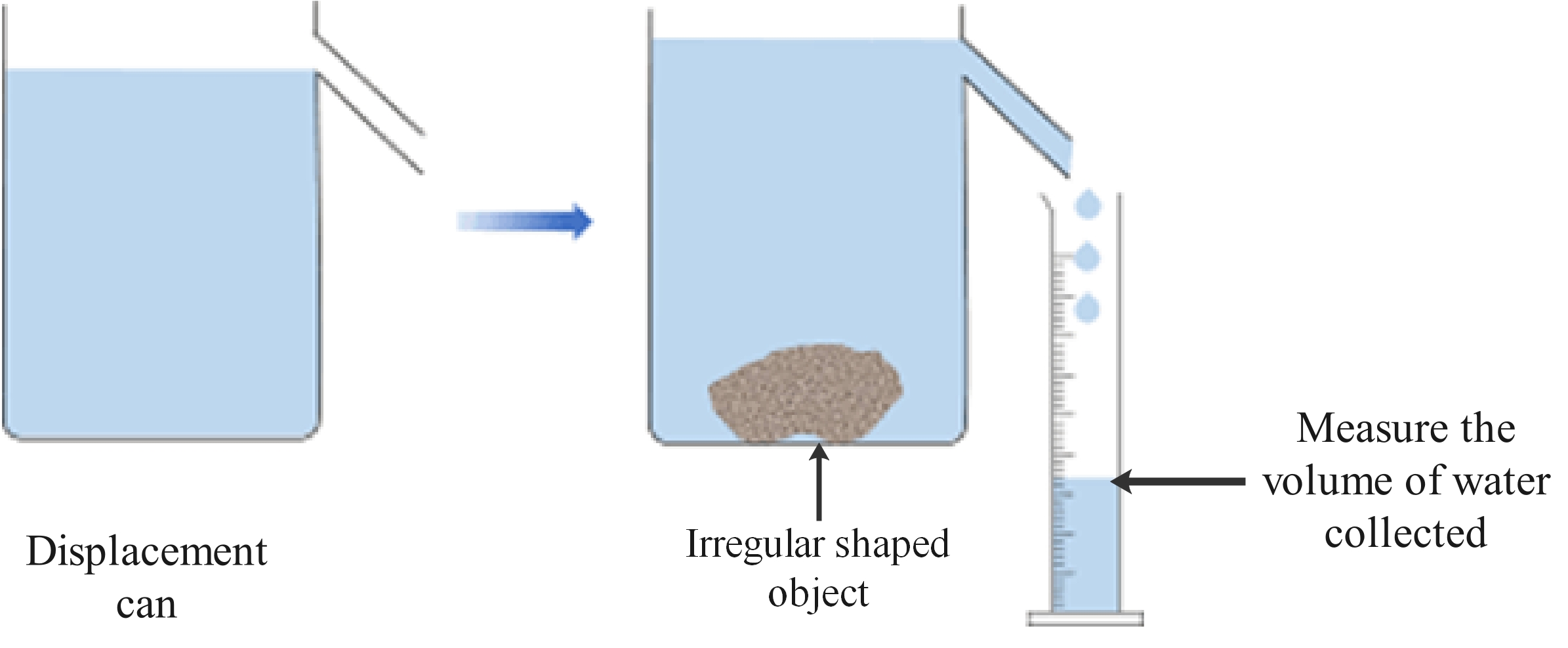
- Place the stone on the mass balance and measure its mass.
- Fill the displacement can until the water is level with the bottom of the pipe.
- Place a measuring cylinder under the pipe ready to collect the displaced water.
- Carefully drop the stone into the can and wait until no more water runs into the cylinder.
- Measure the volume of the displaced water.
- Use the measurements to calculate the density of the stone.
Method 2: Water (or any liquid)
- Place the measuring cylinder on the mass balance and measure its mass.
- Pour 50 cm3 of water into the measuring cylinder and measure its new mass.
- Subtract the mass in step 1 from the mass in step 2. This is the mass of 50 cm3 of water.
- Use the measurements to calculate the density of the water.
Results
Some example results could be:
Object Mass (g) Volume (cm³) Density (g/cm³) Density (kg/m³) Steel cube 468 60 Steel sphere 33 4.19 Stone 356 68 Water 50 50 Analysis
Using those results, the densities can be calculated using:
Density = mass ÷ volume
Density = mass ÷ volume = 33 ÷ 4.19 = 7.9 g/cm3 (= 7,900 kg/m3 )
For a stone of mass 356 g, the volume of water displaced into the measuring cylinder is 68 cm3
Density = mass ÷ volume = 356 ÷ 68 = 5.2 g/cm3 (= 5,200 kg/m3 )
Mass of 50 cm3 of water is found to be 50 g.
Density = mass ÷ volume = 50 ÷ 50 = 1 g/cm3 (= 1,000 kg/m3 )
-
