Section outline
-
-
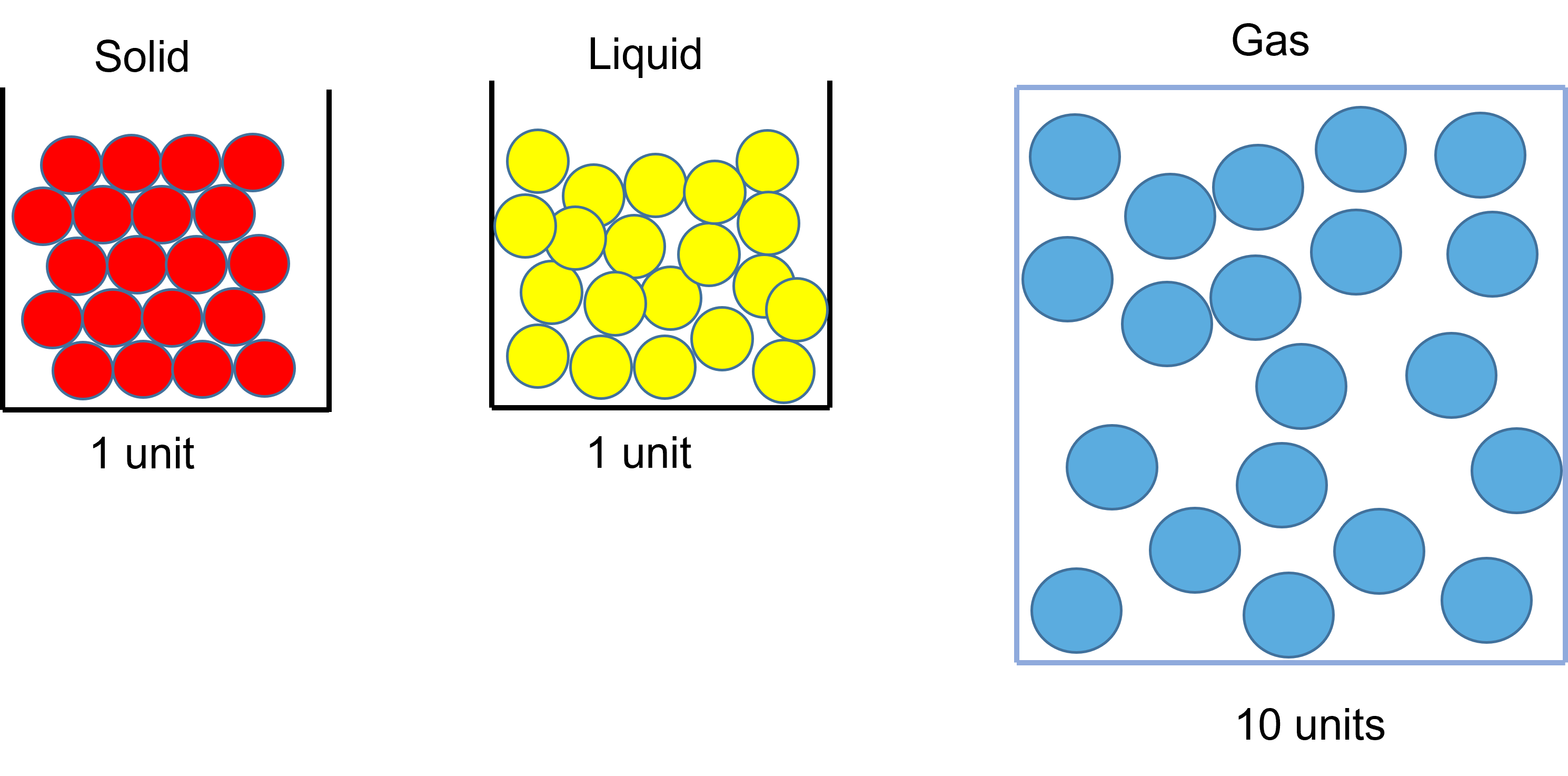
All matter contains particles. The difference between the different states of matter is how the particles are arranged:
- in a solid ‐ particles are tightly packed in a regular structure
- in a liquid ‐ particles are tightly packed but free to move past each other
- in a gas ‐ particles are spread out and move randomly
The density of an object or substance is its mass divided by its volume.
The units of density depend on the units used for mass and volume. The most commonly used units are grams per cubic centimetre (g/cm³) or kilograms per cubic metre (kg/m³).
There is little difference between the density of a liquid and its corresponding solid (eg water and ice). This is because the particles are tightly packed in both states. The same number of particles are spread further apart than in the liquid or solid states. The same mass takes up a bigger volume - this means the gas is less dense.
Density also depends on the material. A piece of iron with the same dimensions as a piece of aluminium will be heavier because the atoms are more closely packed, and because an individual iron atom is heavier than an aluminium atom.
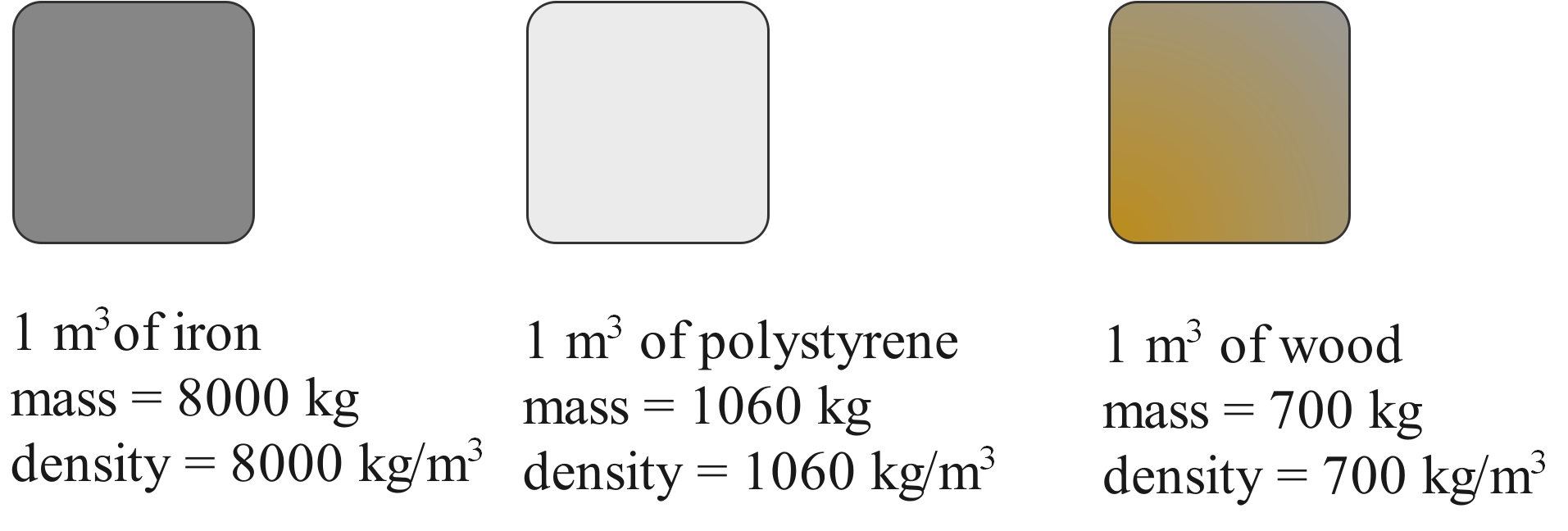
The iron cube is much heavier than the wooden cube, even though they are both the same size. This is because the iron cube has a much higher density. The cube of polystyrene is the same size but is lighter than both because it has an even lower density.
Scientists can measure how tightly packed the particles are by measuring the mass of a certain volume of the material, for example, one cubic centimetre.
Material Density (g/cm³) Iron 7.8 Ice 0.98 Water 1 Air 0.00129
The density of a substance depends both on the mass of the particles, and how closely spaced they are. This means that density changes when substances change state, and when they are heated or cooled.
Calculating density
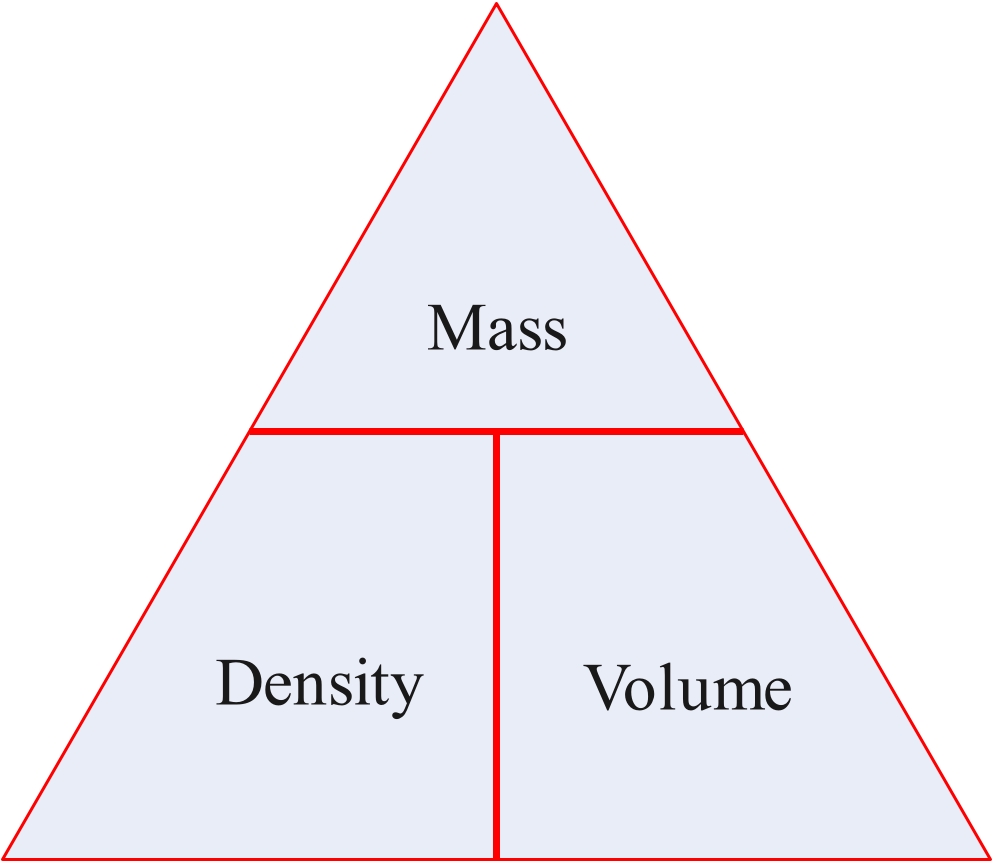
Density = mass/voume
mass = density x volume
volume = mass/density
This is when:
- density (ρ) is measured in kilograms per cubic metre (kg/m3 )
- mass (m) is measured in kilograms (kg)
- volume (V) is measured in cubic metres (m3)
Example
What is the density of a material if 0.45 cubic metres (m3 ) of it has a mass of 0.2 kg?
d = m/v
d= 0.2/0.45
d = 0.44 kg/m3
Question:
What is the density of a material if 4 cubic metres (m3 ) of it has a mass of 2,200 kg?
d = m/v
d = 2200/4
d = 550 kg/m3
The standard unit for mass is kilograms (kg) and for volume is cubic metres (m3). However, in many laboratory situations it is common to measure the mass in grams (g) and volume in cubic centimetres (cm3).
Calculating density using grams and cubic centimetres would give a density unit of grams per cubic centimetre (g/cm³).
1 g/cm3 is equal to 1,000 kg/m3
- To convert from kg/m3 to g/cm3, divide by 1,000.
- To convert from g/cm3 to kg/m3, multiply by 1,000.
Aluminium has a density of 2.7 g/cm3, or 2,700 kg/m3. Lead has a density 11.6 g/cm3, or 11,600 kg/m3.
Example:
Iron has a density of 7.9 g/cm3 what is this in kg/m3 ?
7.9 multiplied by 1,000 gives 7,900 kg/m3.
Question:
What is the density of an object in kg/m3 if it is 6.531 g/cm3 ?
Answer:
6.531 x 1,000 would give 6,531 kg/m3.
Relative density
If placed in water, the iron cube will sink, but the polystyrene and wood cubes will float. This is because the iron cube is more dense than the water and the wood and polystyrene cubes are less dense than water.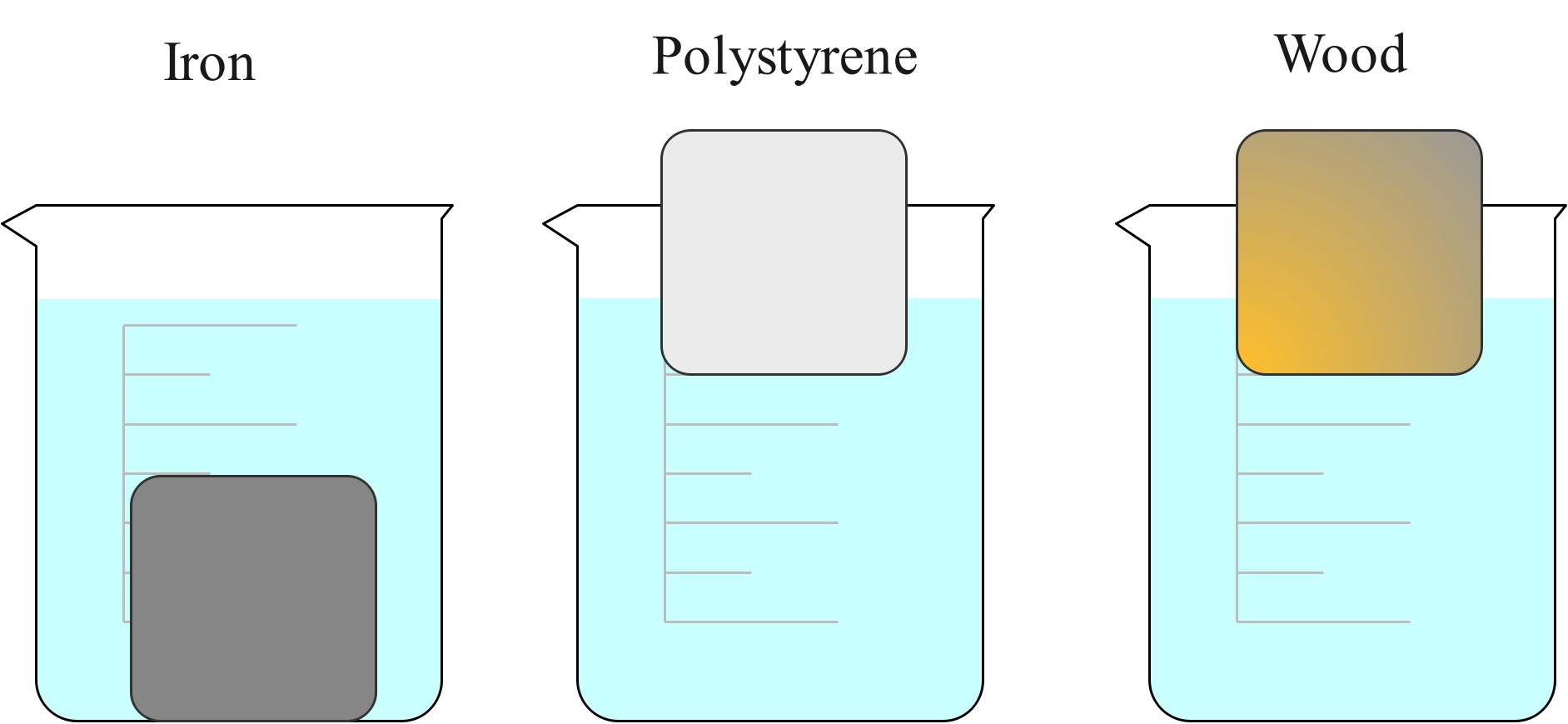
Relative density is the ratio of the density of a substance to the density of water. It is also known as specific gravity. It is a unitless value. If it is less than 1, the substance is less dense than water and would float. If it is greater than 1, the substance is denser than water. If it is exactly 1, the densities are equal. It is usually measured at room temperature and standard atmosphere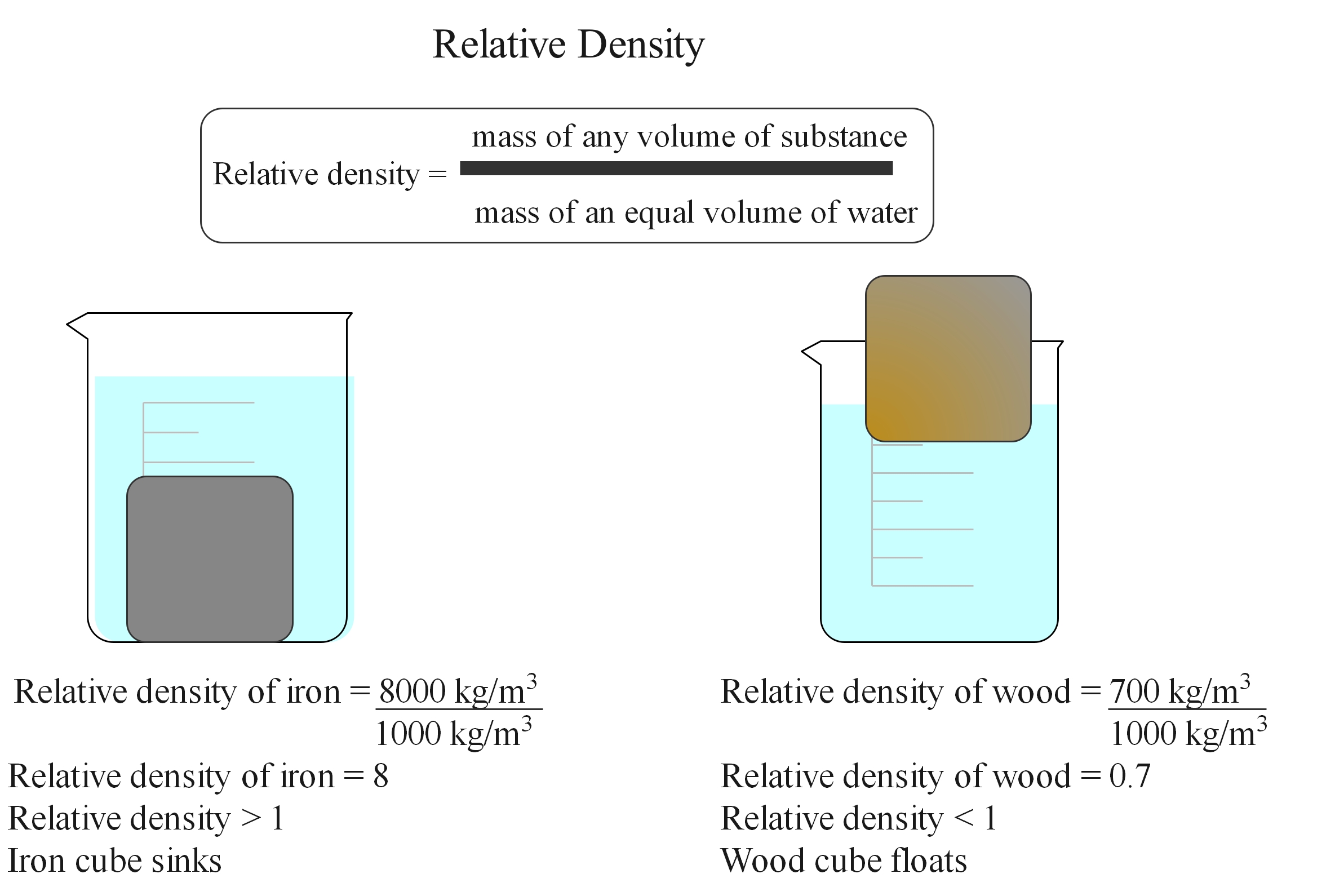
-
