Sectieoverzicht
-
-
Relationship Between Temperature and Kinetic Energy
Kinetic energy is the energy possessed by a body due to its motion. We see a range of kinetic energy in molecules because all molecules don’t move at the same speed. When a substance absorbs heat, the particles move faster, so the average kinetic energy and therefore the temperature increases.
As stated in the kinetic-molecular theory, the temperature of a substance is related to the average kinetic energy of the particles of that substance. When a substance is heated, energy increases the motion of the particles. This will cause an increase in the temperature of the substance.
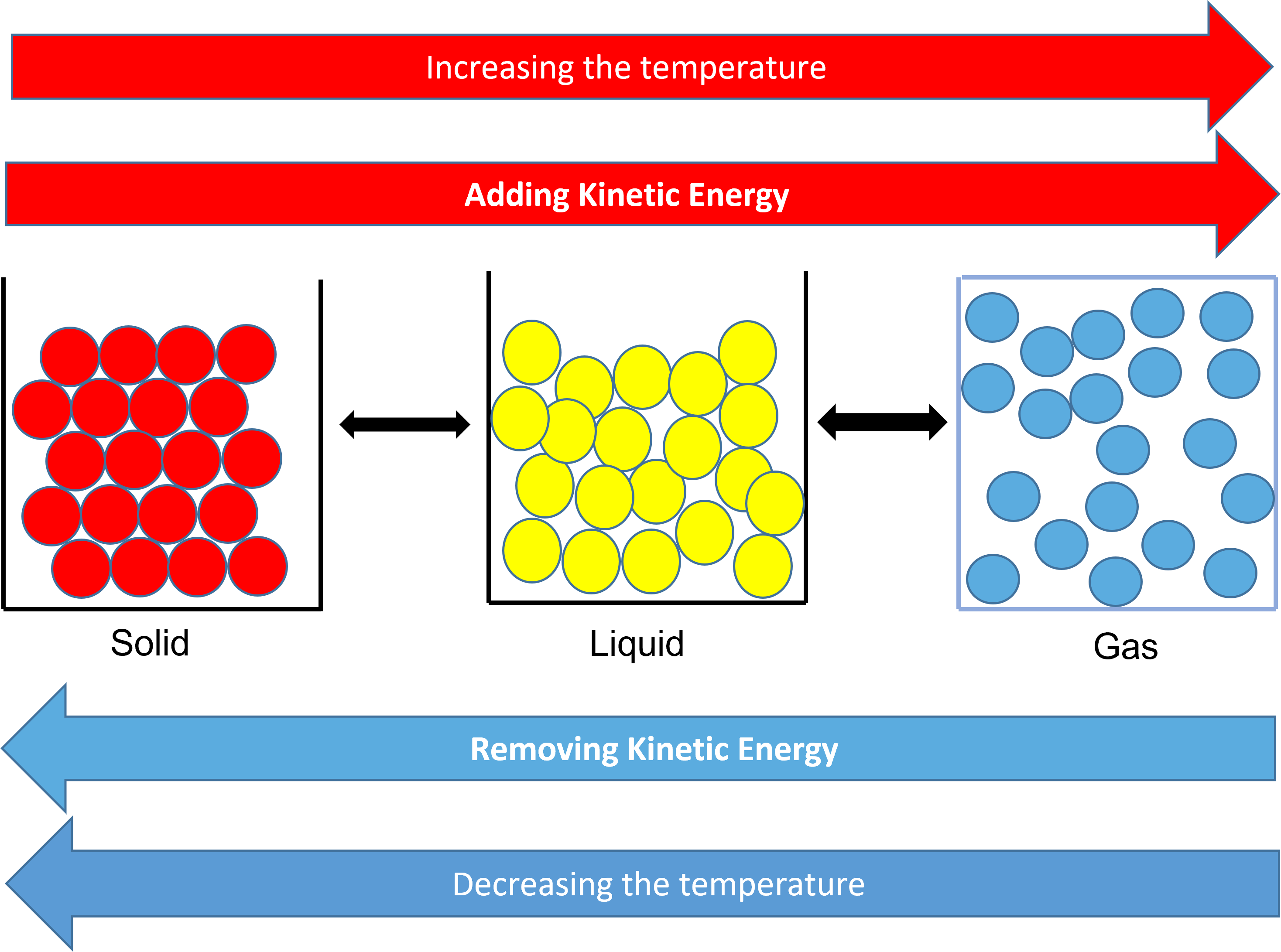
Solids, liquids, and gases all have a temperature. The particles within a solid don’t move, but they vibrate. The temperature increases when molecules vibrate faster. The melting point of a solid is the temperature at which the vibrational motion overcomes the forces of attraction holding the molecules in a solid formation. This is when it changes state. A change of state is a physical property.
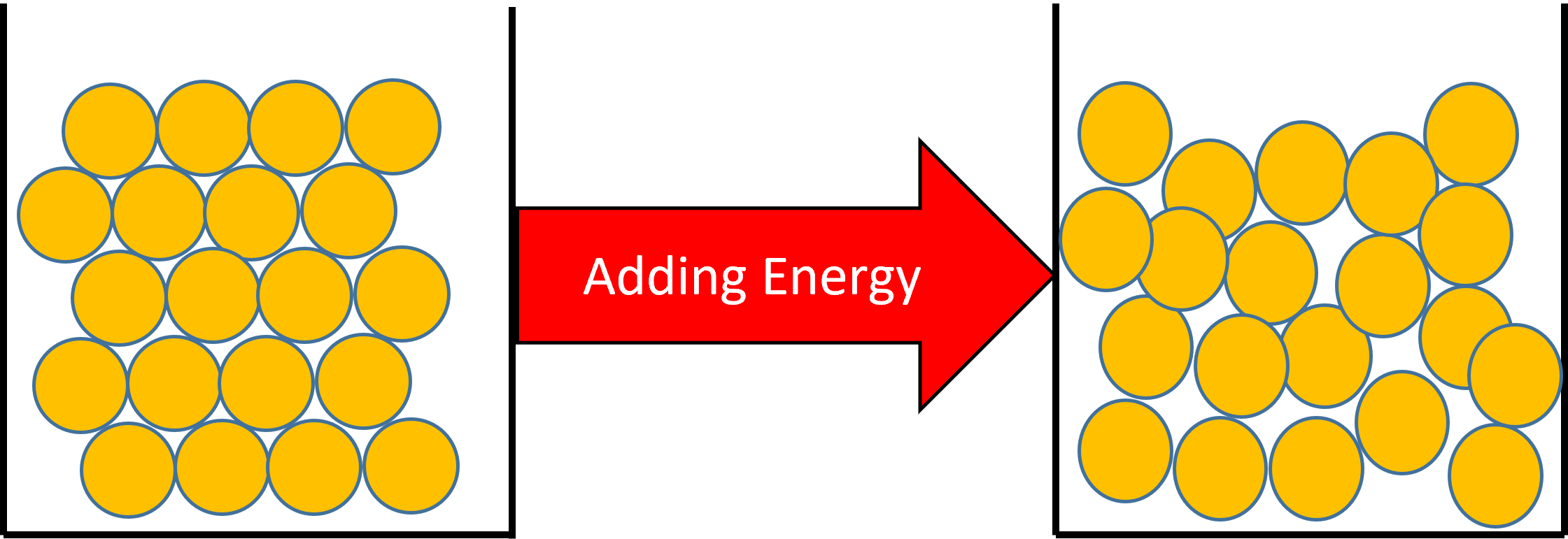
Figure 1: When you add heat to a solid, the particle's kinetic energy will increase. If enough energy is added, the particles in the solid will have enough energy to change their arrangement and become a liquid.
A change of state from a solid to a liquid will have the effect of increasing the substances temperature.
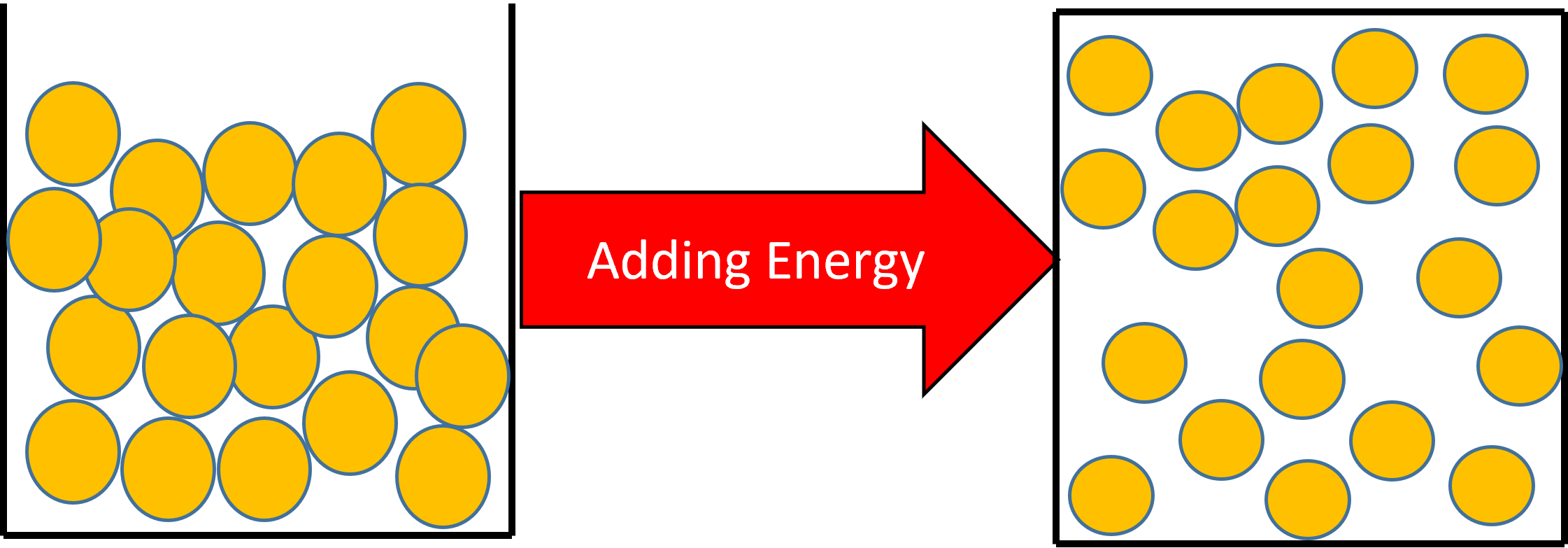
Figure 2: If you continue heating the liquid, the particles will gain more kinetic energy. If enough energy is added, the particles will have enough kinetic energy to change their arrangement and become a gas.
A change of state from a liquid to a gas will have the effect of increasing the substances temperature.
Activity 1
1. Click on the link below:
https://phet.colorado.edu/sims/html/states-of-matter-basics/latest/states-of-matter-basics_en.html
On the home screen press the states tab.

2. Using the menu on the right-hand side, press water icon and the solid icon. What do you notice about the behaviour of the particles?
3. Change the temperature from Kelvin to Celsius. Using the lever on the bucket under the molecules, increase the temperature until it reaches 00 C. What do you notice about the behaviour of the particles?
4. Using the lever, raise the temperature to 1000 C. What do you notice about the behaviour of the particles?
5. Continue to increase the temperature until it reaches approximately 2000C. Has the behaviour of the particles changed?
6. Using the lever, decrease the temperature and observe the changes in behaviour.
7. Reduce the temperature to the lowest possible temperature this should be somewhere close to 274K. How is the behaviour different than when the temperature was close to 00C?
Conclusion
As you increase the temperature, the particles gain more kinetic energy. This allows them to change state.
When you reduce the temperature, the particles loose kinetic energy and again they change state.
When water is near to absolute zero, the water particles, in the solid state of ice, do not even vibrate.
-
